Modifying E. Coli to Diagnose — and Treat — Colorectal Cancer
Researchers design new approach that could lead to simpler, non-invasive treatments in the future.
Media Contact
Holly Evarts, Director of Strategic Communications and Media Relations 347-453-7408 (c) | 212-854-3206 (o) | [email protected]
About the Study
JOURNAL: Nature Communications
TITLE: "Engineering tumor-colonizing E. coli Nissle 1917 for detection and treatment of colorectal neoplasia"
COLLABORATORS: Columbia Engineering; Columbia University; Columbia University Vagelos College of Physicians and Surgeons; The South Australian Health and Medical Research Institute; Adelaide Medical School at University of Adelaide; Weill Cornell; Royal Adelaide Hospital; College of Medicine and Public Health at Flinders University; and University of South Australia-Adelaide
FUNDING: This work was supported in part by the National Institute of Health U01CA247573 (T.D.), National Institute of Health R01CA24916 (T.D.), the National Science Foundation Graduate Research Fellowship (1644869 to C.R.G.), Pershing Square Foundation (PSF) PSSCRA CU20-0730 (T.D.), Cancer Research Institute (CRI) CRI 3446 (T.D.) National Health and Medical Research Council (APP1184925 to S.L.W.), Cancer Council SA Beat Cancer Project on behalf of its donors and the State Government of South Australia through the Department of Health (MCF0418 to S.L.W.), the Gastroenterological Society of Australia Bushell Post-doctoral Research Fellowship (S.L.W.), the Faculty of Health Science at the University of Adelaide (S.L.W.), the South Australian Health and Medical Research Institute (S.L.W.).
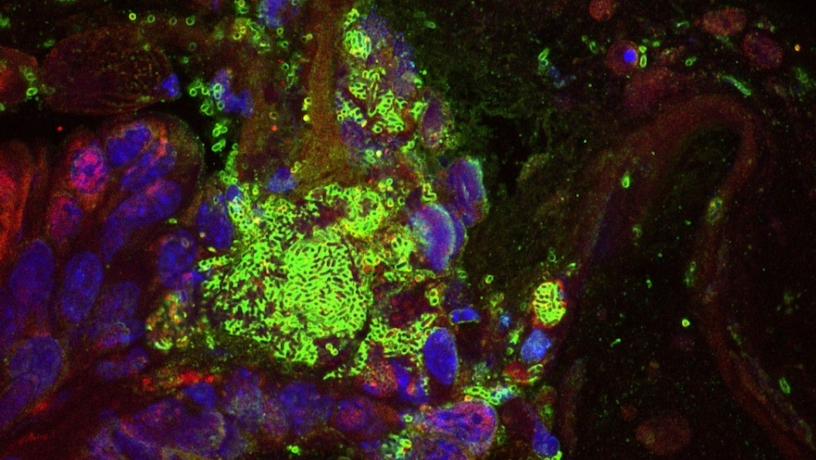
Immunofluorescent staining of colorectal cancer tumor bearing mouse fed engineered E. coli Nissle 1917 (EcN). Image depicts the location of engineered EcN. Staining includes Hypoxyprobe (red) and lipopolysaccharide (LPS, green, scale bar 10um). Credit: Susan Woods/Adelaide Medical School and South Australian Health and Medical Research Institute
Colorectal cancer (CRC) is a leading cause of cancer-related deaths, with 10% of new CRC cases arising in people under age 50. It is predicted that young-onset CRC could be the deadliest cancer by 2030 in people aged 20-49. Diagnosing CRC early is key to successful treatment. Colonoscopies are the current method for diagnosis of CRC, but are unpleasant and expensive — as a result, many young people don’t get them.
Earlier this month, an international team of researchers led byTal Danino, associate professor of biomedical engineering at Columbia Engineering, published a paper in Nature Communications laying the foundation for a new approach to orally diagnose and treat CRC using genetically modified bacteria -- even in the form of a pill.
Danino, along with his collaborator, Nicholas Arpaia and the Arpaia lab at Columbia’s Vagelos College of Physicians and Surgeons, have for years focused on engineering bacteria as a novel way to identify and attack tumors. In October, the researchers engineered tumor-colonizing bacteria (probiotics) to produce synthetic targets in tumors that direct CAR-T cells to destroy targeted cancer cells. The team has also previously developed bacteria that deliver immunotherapy payloads, and they continue to refine their work with the aim of assessing the technique’s safety and efficacy in human patients.
Bioengineered probiotics for the treatment and prevention of colorectal cancer
A “good” kind of E. coli
E. coli Nissle 1917 (EcN) — a probiotic microbe that typically fights against harmful gut bacteria — also thrive in colorectal cancer tumor cores.
Chemical alarm bells
Researchers used genetic engineering to modify EcN to produce a small molecule called salicylate. When this engineered EcN strain colonizes cancerous cells, the bacteria flourish and produce high levels of salicylate, a molecule that marks the presence of cancer lesions.
An innovative cancer test
In the body, the salicylate produced by EcN is filtered out through the urine. In pre-clinical studies led by Candice Gurbatri, PhD student in the Danino lab at Columbia Engineering, mice with precancerous lesions in the colon orally fed this modified EcN had much higher salicylate levels in their urine, compared to healthy mice. This suggests that testing the urine for salicylate could be used to determine the presence of colorectal cancer.
A potential treatment
The researchers then engineered EcN to produce cancer-fighting molecules, like cytokines and immunotherapeutic payloads. When mice with colorectal cancer were fed these strains, their tumors shrank by about 50%. Validating their approach in human patients, the team, which included Susan Woods from the Adelaide Medical School and South Australian Health and Medical Research Institute (SAHMRI), Daniel Worthley (SAHMRI), and others, conducted an interventional, double-blind, dual-center, prospective clinical trial demonstrating that unengineered forms of EcN could colonize human colorectal tumors.
The path forward
Together, these results support the use of EcN as a platform to detect and treat CRC — in the form of a pill. Next steps would involve testing engineered forms of EcN in human patients to validate both diagnostic and therapeutic approaches.
“Oral delivery of engineered probiotics is a promising approach for detecting and treating colorectal cancer, and we plan to expand on this work in the future,” said Danino.
Read the abstract
Bioengineered probiotics enable new opportunities to improve colorectal cancer (CRC) screening, prevention, and treatment. Here, first, we demonstrate selective colonization of colorectal adenomas after oral delivery of probiotic E. coli Nissle 1917 (EcN) to a genetically engineered murine model of CRC predisposition and orthotopic models of CRC. We next undertake an interventional, double-blind, dual-center, prospective clinical trial, in which CRC patients take either placebo or EcN for two weeks prior to resection of neoplastic and adjacent normal colorectal tissue (ACTRN12619000210178). We detect enrichment of EcN in tumor samples over normal tissue from probiotic-treated patients (primary outcome of the trial). Next, we develop early CRC intervention strategies. To detect lesions, we engineer EcN to produce a small molecule, salicylate. Oral delivery of this strain results in increased levels of salicylate in the urine of adenoma-bearing mice, in comparison to healthy controls. To assess therapeutic potential, we engineer EcN to locally release a cytokine, GM-CSF, and blocking nanobodies against PD-L1 and CTLA-4 at the neoplastic site, and demonstrate that oral delivery of this strain reduces adenoma burden by ~50%. Together, these results support the use of EcN as an orally-deliverable platform to detect disease and treat CRC through the production of screening and therapeutic molecules.